Acid Mucopolysacchardies Research
CHAPTER VI from: Lester M. Morrison: CORONARY HEART DISEASE AND THE MUCOPOLYSACCHARIDES, 1974. Reprinted with permission, Charles C. Thomas, Publisher, Ltd., Springfield Illinois
IN CONSIDERING the mechanism of effect of any therapeutic substance on cellular systems in vivo it is important to know (a) whether the substance actually reaches the cells which show the response, (b) whether it enters the cells in whole or in part, and (c) if the substance enters the cells, the details with respect to the form in which it exists therein.
These questions hover with a special urgency over the heads of those investigating the actions of the acid mucopolysaccharides. This is because the molecules are sufficiently large so that it might be questioned whether they enter cells (other than phagocytes) intact even if they are injected into the circulation; and further, because it has been found that small units (of about 6,000 in molecular weight), at least in the case of chondroitin-4-sulfate and chondroitin polysulfate, are without biologic effect effect in certain of the systems tested. These questions become even more critical when it is considered that such acid mucopolysaccharides as the chondroitin sulfates appear to yield results when administered orally. If this is true they would be expected to be found in significant amounts in the circulating blood and in urine following ingestion.
Acid mucopolysaccharides are secreted from the cell in vesicles (exocytosis, see Chapter III), end it is to be suspected that what can leave by this means call also enter by the reverse of the process (pinocytosis). The major difficulty in logically pursuing this line of thought is that the acid mucopolysaccharides resident in the areas among connective tissue cells arrived there in association with protein. From the work of De Robertis and Vaz Ferriera1 and others2,3, it can be suspected that reverse pinocytosis of noproteinaceous substances usually takes place when the material being excreted is associated with protein. Moreover, pinocytosis (the carrying of macro-molecules inward from the surface of the cell within membrane vesicles) is often quite specific with respect to the type of protein admitted. Brambell et al.4 have shown that a limited portion of the protein may hold the key for entry, and Schieide et al.5 have provided evidence that only certain parts of the plasma membrane will invaginate to allow entry of specific protein species.
In the case of the chondromucoproteins, however, even those which are excreted from the cell probably present a largely nonproteinaceous (albeit highly negatively charged) surface to the membranes with which they come into contact. The protein moiety of the chondromucoprotein constitutes only approximately 7 percent of the total molecule and can be envisioned as a somewhat elongated pincushion from which protrude a dozen or more long polysaccharide strands (see Chapter II). Antibodies can be formed against the various chondro-mucoproteins6 but these may result from exposure within the antibody-forming cells to the protein moiety following alteration of the complexes.
As will be detailed in the sections which follow, it appears likely that acid mucopolysaccharides of high molecular weight, even when not associated with protein, are taken into at least certain cells if injected intravenously or subdermally and that entry into the circulation can he effected when the material is taken orally.
It is tempting to speculate on the possibility of a special interrelationship between a substance presenting such an extensive array of negative charges and cellular membranes. Some experimental evidence relating to this notion is provided by Feldhert7,8 who coated gold particles of about 150 D diameter with polyvinylpyrrolidone which imparts multiple negative charges to the particles. These entities, as well as highly positively charged particles, were observed in the electron microscope to traverse the nuclear annular pores (when introduced into amoeba) by a process closely resembling pinocytosis.9,10 On the other hand, neutrally charged gold particles aligned themselves with the annular rods constituting the peripheral cylinders of the nuclear pores but failed to pass into the nucleus. It is possible that some relatively nonspecific permeases (proteins which facilitate entry into the cell of molecules ranging up to the size of viruses11) are involved in this mechanism.
Chondroitin-4-sulfate-35S was biosynthetically prepared by intraperitoneal injection of sodium 35SO4 in suckling rats followed by extraction and purification of labeled chondroitin sulfate by the method of Dziewiatkowski.14 The labeled chondroitin-4-sulfate (50 mg) was administered orally to patients together with cold chondroitin-6-sulfate (950 mg) and the output pattern in urine was examined for thirty days. The maximal output of 35S appeared in urine the second day following administration (see Fig. 17). Approximately three fourths of the radioactivity was excreted during the first forty-eight hours and nearly 90 percent had been excreted after ninety-six hours. Traces of activity were observed during the following thirty days. A non-dialyzable high molecular weight substance with 35S activity accounted for 8 percent of the total 35S count in the urine. This observation indicates that chondroitin-4-sulfate was absorbed from the human intestine and was excreted in urine mostly in the form of desulfated smaller molecules. Nevertheless, some small amounts of high molecular weight chondroitin-4-sulfate appeared in the urine.
In studies carried out on animals, 16 milligrams of 35S chondroitin-4-sulfate and 3.2 milligrams of 14C chondroitin- 4-sulfate were given orally to mice. About 40 percent of the 35S chondroitin-4-sulfate was absorbed within the first twenty-four hours. The ratio of absorbed material to nonabsorbed material (that remaining in the gastrointestinal tract and feces) was approximately 1:2 within twenty-four hours. The urine contained 48 percent of the 35S activity of the absorbed chondroitin sulfate. 14C chondroitin-4-sulfate was also absorbed from the intestine within twenty-four hours. Again, in the case of the 14C radioisotope, the ratio of the absorbed to nonabsorbed materials was approximately 1:2. Furthermore, high molecular weight isotope-labeled chondroitin-4-sulfate was detected in blood and in urine by electrophoretic procedures.15
Administration of chondroitin polysulfate to animals was by the oral route at the rate of 4.8 milligrams per grams of body weight in rats and 2.0 milligrams per gram of body weight in dogs. The concentrations of chondroitin polysulfate appearing in the blood were determined by denaturing the proteins, treating the denatured proteins with proteases and then subjecting the clear supernatant to hexuronic acid determination.16 By means of the above techniques, it was found that the concentration of whole chondroitin polysulfate in the blood increased rapidly during the first ninety minutes in rats and during the first thirty minutes in dogs following oral administration. It then decreased gradually but remained at relatively high levels for six hours in rats and ten hours in dogs (see Fig. 18). Absorption from isolated intestinal segments of rabbits was examined by placing either 50 or 200 milligrams of chondroitin polysulfate into a section of intestine and perfusing with blood. In the case of either aliquot, maximum absorption rates were observed between fifty and sixty minutes and the accumulative absorption of chondroitin polysulfates in sixty minutes amounted to 13 and 19 percent with 50 and 200 milligrams respectively.17 3H- and 35S-labeled chondroitin polysulfates, considered to be nearly identical in terms of molecular weight (6,000 daltons) and other parameters, were prepared. These labeled chondroitin polysulfates were purified by passing them through a Dowex 1-X2 column and Sephadex G-25. The 3H chondroitin polysulfate thus obtained possessed 14.2 percent sulfur by weight and 1.47 X 107 dpm/mg (distribution per minute/ milligram) of specific activity. The 35S substance had 15.3 percent sulfur and its specific activity was 8.09 X 104 dpm/mg. Electrophoresis and fractionation on Sephadex indicated uniform disribution of molecular weight and sulfur content.18
The 3H-labeled chondroitin polysulfate was given orally (400 mg/kg) to both Wistar male rats and to NewZealand male rabbits. At appropriate intervals, blood and urine samples were taken for the purpose of measuring absorbed radioactivity and breakdown products of chondroitin polysulfate. The concentrations and activities of the substances in plasma were determined by denaturing proteins (heating) and and subjecting the denatured proteins to proteolytic digestion. A filtrate was passed through a Dowex 50-X2 column (H+) and neutralized with alkali solution. Lipids were extracted with chloroform methanol.
In Figure 19, the 3H radioactivity and localization of 3H chondroitin polysulfate in paper electrophoretic patterns of rat serum at two hours after oral administration are shown.19 The fact that significantly high activity was found in an electrophoretic band corresponding to the electrophoretic mobility of 3H chondroitin polysulfate indicates that the substance was absorbed intact from intestine to circulation. Serum lipid clearing activity, measured by free fatty acid content and turbidity also appeared to be related to absorption. As can be seen in figure 20, this physiolosical activity of chondroitin polysulfate affords additional proof that chondroitin polysulfate is absorbed from the intestine.20
In figure 21, the specific radioactivity in rabbit serum measured at the indicated hours after oral administration of 3H chondroitin polysulfate is shown. The blood level of radioactivity increased rapidly within the first two hours, then rose gradually to a maximum at eight hours and decreased over a period of approximately sixteen hours.21 In urine, on the other hand, a cumulative excretion of radioactivity following oral administration of 3H chondroitin polysulfate was seen.22
In a study of uptake of orally administered chondroitin sulfates in human subjects, Morrison and his co-workers23 fed individuals of a group of nine patients, displaying active symptomatic coronary heart disease in the form of angina pectoris, 10 grams of chondroitin-4-sulfate each day over a period of three days. (The dosages of chondroitin-4-sulfate were divided into three parts and were taken with meals.) Accumulated acid mucopolysaccharides excreted in the urine were measured each twenty-four hours following the initial ingestion of chondroitin-4-sulfate and were compared to a three-day baseline of urinary excretion established prior to ingestion of test polysaccharide. As can be seen in Table XVII, an increased excretion of acid mucopolysaccharides via the urine followed oral ingestion of chondroitin-4-sulfate. The average excretion of acid mucopolysaccharides during a twenty-four hour period was found to be 5.1 milligrams before patients received supplementary chondroitin-4-sulfate. During the period that 10 grams of the substance was being ingested per day, urinary excretion of total whole acid mucopolysaccharide rose to 7.3 milligrams for a twenty-four hour interval. Tests for significant differences between excretion amounts before and after administration of chondroitin-4-sulfate reveal a P value greater than 0.01.
Chondroitin polysulfate was also given orally (10 grams) to three normal subjects and the output of urinary acid mucopolysaccharides was examined over a period of seventy-two hours. The results of electrophoresis and Dowex column fractionation revealed that the increased acid mucopolysaccharide was excreted in the form of the administered chondroitin polysulfates as well as desulfated and degraded products.24
It is not clear what proportion of the ingested mucopolysaccharide was present inside the cells per se, but detection of the polysaccharides in the blood and urine suggests that they must have at least reached tissues and organs, and traversed glomerular cells in order to reach this latter compartment.
Results shown in Figure 22 are indicative of the metabolism of 35S chondroitin polysulfate in whole rat liver tissue after oral administration.29 Pronase-digested 35S mucopolysaccharides appeared in the liver within six hours but the concentration increased up to twenty-four hours when the activity of large molecular mucopolysaccharides (peak I) was predominant. At forty-eight hours, the activity of 35S was somewhat greater in peak II than in peak I.
Fractionation of urinary mucopolysaccharide preparations (obtained from rabbits receiving 35S chondroitin polysulfate) on Sephadex G-50 provided evidence of a depolymerizing action as a function of duration of metabolism.30 As can be seen in Figure 23, urine specimens taken between zero and five hours show that the larger ratio of activity appears in a high molecular fraction (A). However, the proportion of activity changes between five and eleven hours with a result that higher activity is now present in low molecular weight fractions (E, D and C). The 35S activity in each fraction diminishes gradually until only a single peak (F) is labeled from thirty-six to forty-eight hours.
The above observations reveal the presence in urine of partially degraded as well as whole chondroitin polysulfates, radioactive inorganic sulfate and traces of other low molecular weight consituents (containing 35S) which are probably degadation products of chondroitin polysulfates.31
Gel filtration patterns of twelve-hour cumulative urine in rats following oral administration of 3H chondroitin polysulfate are depicted in figure 24. The highest radioactivities appeared in the void volume of Sephadex G-25 where intact high molecular chondroitin polysulfate is present. However, relatively large amounts of partially degraded substances exhibiting lower radioactivities were also detected.
In tracer studies in which labeled chondroitin sulfates were employed in vivo, various amounts of degradation products were demonstrated as the result of catabolic processes. No exact explanation, however, regarding the fate of the degraded products can be presented at this time. In considering the metabolism of mucopolysaccharides, re-utilization of at least a proportion of the degraded products cannot be ruled out.
Thus far, it remains to be unequivocally that chondroitin sulfates actually enter all cells and if they do, whether some cells are more receptive to them than others. In this regard, it is pertinent to point out that even though biological effects are observed with both injected and orally administered chondroitin sulfates, this is no reason for assuming that they enter all of the cells showing reactions to them. In the scheme of bone induction, as experimentally elucidated by Urist and others,37 it appears that the inducing agent is probably a complex of mucopolysaccharides in associntion with a bone tropocollagen which never enters the presumptive osteoblasts. All of the evidence (including labeling with radioactive materials) indicates that no uptake of the inducing agent by the induced cell takes place.38 In this case the induction process appears to consist of contact of the inducing agent with the surface of a cell which is differentiated in the direction of responding to such a stimulus.
It is, of course, neccessary to learn the nature of the effects of mucopolysaccharidess at the intracellular level if controlled and intelligent therapy is to be applied. For this reason, much important study remains to be carried out with respect to the possible entry of chondroitin sulfates into various cell types, how they enter, if they do and the reactions which occur in the cell thereafter, especially at the level of the genome. Investigations involving the use of radioactive chondroitin-4-sulfate, etc., in tissue cultures and other systems, followed by cell fractionation and analysis of such fractions, would appear to be useful in this regard, as would studies involving radioautography at the level of the electron microscope.
2H. Blaschka, A.D. Smith, H. Winkler, H. Van den Borsch, and L.L. Van Deene, Acid phospholipase A in lysosomes of the bovine adrenal medulla. Biochem J, 103:30-32c, 1967.
3F.H. Schneider, A.D. Smith, and H. Winkler, Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol, 31:94, 1967.
4F.W.R. Brambell, W.A. Hemmings, C.C. Oakley, and R.R. Porter, The relative transmission of the fractions of papain hydrolyzed homologous globulin from the uterine cavity to the portal circulation of the rabbit. Proc R Soc Lond (Biol), 151:478, 1960.
5O.A. Schjeide, F. Galey, E.A. Grellert, R. I-San-Lin, J. de Vellis, and J.F. Meade, Macromolecles in oocytes maturation. Biol Reprod (supp),2:14, 1970.
6C.P. Triganoo, and H. Muir, Studies on protein polysaccharides from pig laryngeal cartilage. Hetrogeneity, fractionation and characterization. Biochem J, 113:855, 1969.
7C.M. Feldherr, Nucleocytoplasmic exchanges during cell division. J Cell Biol, 31:199, 1966.
8C.M. Feldherr, The effect of electron-opaque pore material on exchanges through the nuclear annuli. J Cell Biol, 31:199, 1966.
9Feldherr, The effect of the electron opaque pore material on exchanges through the nuclear annuli.
10Feldherr, Nucleocytoplasmic exchanges during cell division.
11L.B. Crittenden, W.E. Briles, and H.A. Stone, Susceptability to an avian lukosis-sarcoma virus: a close association with erythrocyte isoantigen. Science, 59:1324, 1970.
12K. Murata, M. Nomato, and T. Funaki, In preparation.
13F. Haruki, K. Murata, and H. Debuchi, Further studies on the metabolism of chondroitin sulfate. Jap Rheum, 2:453, 1960.
14D.D. Dziewiatowski, Some aspects of the metabolism of chondroitin sulfate-35S in the rat. J Biol Chem,223:239, 1956.
15K. Murata, Chondroitin sulfates in clinical aspects. In Biochemistry of Acid Mucopolysaccharides, S. Suzuki, T. Matsumura, and I. Yamashina, Eds. (Tokyo, Kyoritsu, 1970), vol. II, p. 1140.
16T. Ofuji, and T. Funaki, Absorption and excretion of sodium chondroitin polysulfate. In preparation.
17Ibid.
18Murata, Nomoto, and Funaki.
19Ibid.
20Ibid.
21Ibid.
22Ibid.
23L.M. Morrison, P.G. Rucker, and M.R. Stevens, Unpublished observations.
24T. Ogura, S. Suzuki, and T. Yamaguchi, Studies on urinary mucosubstances following chondroitin polysulfate administration in men. In preparation.
25Murata, Nomoto, and Funaki.
26Haruki, Murata, and Debuchi.
27M.L. Salkie, The acid glycosaminoglycan-degrading activity of normal human serum. Clin Chim Acta, 31:300, 1971.
28N.N. Aronson, Jr., and E.A. Davidson, Catabolism of mucopolysaccharides by rat liver lysosome in vivo. J Biol Chem, 243:4494, 1968.
29Murata, Nomoto, and Funaki
30Ibid.
31Ibid.
32Ibid.
33D. Kaplan, and K. Meyer, The fate of injected mucopolysaccharides. J Clin Invest, 41:743, 1962.
34Haruki, Murata, and Debuchi.
35Murata, Nomoto, and Funaki.
36Ibid.
37M.R. Urist, Induction and differentiation of cartilage and bone cells. In: Cell Differentiation, O.A. Schjeide, and J. De Vellis, Eds. (New York, Van Nostrand Reinhold, 1970), p.504.
38Ibid.
ABSORPTION, DISTRIBUTION, METABOLISM AND EXCRETION OF ACID MUCOPOLYSACCHARIDES ADMINISTERED TO ANIMALS AND PATIENTS
Co-authored by Katsumi Murata M.D., Ph.D., Department of Medicine and Physical Therapy. University of Tokyo School of Medicine.IN CONSIDERING the mechanism of effect of any therapeutic substance on cellular systems in vivo it is important to know (a) whether the substance actually reaches the cells which show the response, (b) whether it enters the cells in whole or in part, and (c) if the substance enters the cells, the details with respect to the form in which it exists therein.
These questions hover with a special urgency over the heads of those investigating the actions of the acid mucopolysaccharides. This is because the molecules are sufficiently large so that it might be questioned whether they enter cells (other than phagocytes) intact even if they are injected into the circulation; and further, because it has been found that small units (of about 6,000 in molecular weight), at least in the case of chondroitin-4-sulfate and chondroitin polysulfate, are without biologic effect effect in certain of the systems tested. These questions become even more critical when it is considered that such acid mucopolysaccharides as the chondroitin sulfates appear to yield results when administered orally. If this is true they would be expected to be found in significant amounts in the circulating blood and in urine following ingestion.
Acid mucopolysaccharides are secreted from the cell in vesicles (exocytosis, see Chapter III), end it is to be suspected that what can leave by this means call also enter by the reverse of the process (pinocytosis). The major difficulty in logically pursuing this line of thought is that the acid mucopolysaccharides resident in the areas among connective tissue cells arrived there in association with protein. From the work of De Robertis and Vaz Ferriera1 and others2,3, it can be suspected that reverse pinocytosis of noproteinaceous substances usually takes place when the material being excreted is associated with protein. Moreover, pinocytosis (the carrying of macro-molecules inward from the surface of the cell within membrane vesicles) is often quite specific with respect to the type of protein admitted. Brambell et al.4 have shown that a limited portion of the protein may hold the key for entry, and Schieide et al.5 have provided evidence that only certain parts of the plasma membrane will invaginate to allow entry of specific protein species.
In the case of the chondromucoproteins, however, even those which are excreted from the cell probably present a largely nonproteinaceous (albeit highly negatively charged) surface to the membranes with which they come into contact. The protein moiety of the chondromucoprotein constitutes only approximately 7 percent of the total molecule and can be envisioned as a somewhat elongated pincushion from which protrude a dozen or more long polysaccharide strands (see Chapter II). Antibodies can be formed against the various chondro-mucoproteins6 but these may result from exposure within the antibody-forming cells to the protein moiety following alteration of the complexes.
As will be detailed in the sections which follow, it appears likely that acid mucopolysaccharides of high molecular weight, even when not associated with protein, are taken into at least certain cells if injected intravenously or subdermally and that entry into the circulation can he effected when the material is taken orally.
It is tempting to speculate on the possibility of a special interrelationship between a substance presenting such an extensive array of negative charges and cellular membranes. Some experimental evidence relating to this notion is provided by Feldhert7,8 who coated gold particles of about 150 D diameter with polyvinylpyrrolidone which imparts multiple negative charges to the particles. These entities, as well as highly positively charged particles, were observed in the electron microscope to traverse the nuclear annular pores (when introduced into amoeba) by a process closely resembling pinocytosis.9,10 On the other hand, neutrally charged gold particles aligned themselves with the annular rods constituting the peripheral cylinders of the nuclear pores but failed to pass into the nucleus. It is possible that some relatively nonspecific permeases (proteins which facilitate entry into the cell of molecules ranging up to the size of viruses11) are involved in this mechanism.
A. Evidence of Absorption From the Intestine
Studies of absorption of chondroitin sulfates as well as chondroitin polysulfates from the digestive tract have been carried out cooperatively by Dr. Kitsumi Murata of the University of Tokyo and Dr. Masao Nomoto and Dr. T. Funaki at their institutes.12 These investigators used radioisotope-labeled chondroitin sulfates and chondroitin polysulfates (sulfation was synthetically carried out on chondroitin-6-sulfate obtained from shark cartilage) in studies of uptake in tissue and excretion in urine. They also measured uptake of 35S-labeled chondroitin-4-sulfate (obtained from suckling rat costal and knee joint cartilage) and nonlabeled chondroitin-6-sulfate in patients at the University of Tokyo School of Medicine.13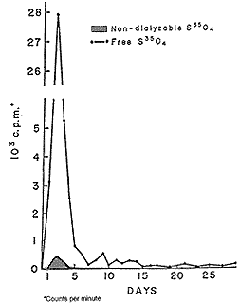 |
| Figure 17. Excretion profile of S35-labeled chondroitin sulfate A in man after oral administration in man. |
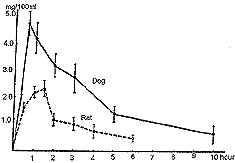 |
| Figure 18. Serum uronic acid levels following oral administration of chondroitin polysulfate. Dosage levels were 4.8 mg/g in rats (five animals) and 2.0 mg/g in dogs (five animals). |
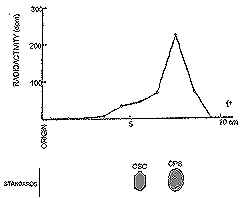 |
| Figure 19. Paper electrophoresis of proteinase digests of rat serum to hours following oral administration of 3H chondroitin polysulfate. |
Chondroitin-4-sulfate-35S was biosynthetically prepared by intraperitoneal injection of sodium 35SO4 in suckling rats followed by extraction and purification of labeled chondroitin sulfate by the method of Dziewiatkowski.14 The labeled chondroitin-4-sulfate (50 mg) was administered orally to patients together with cold chondroitin-6-sulfate (950 mg) and the output pattern in urine was examined for thirty days. The maximal output of 35S appeared in urine the second day following administration (see Fig. 17). Approximately three fourths of the radioactivity was excreted during the first forty-eight hours and nearly 90 percent had been excreted after ninety-six hours. Traces of activity were observed during the following thirty days. A non-dialyzable high molecular weight substance with 35S activity accounted for 8 percent of the total 35S count in the urine. This observation indicates that chondroitin-4-sulfate was absorbed from the human intestine and was excreted in urine mostly in the form of desulfated smaller molecules. Nevertheless, some small amounts of high molecular weight chondroitin-4-sulfate appeared in the urine.
TABLE XVI: AMOUNTS OF CHONDROITIN-4-SULFATE ABSORBED FROM MOUSE INTESTINE
| S-labeled chondroitin-4-sulfate | C-labeled chondroitin-4-sulfate | |||||
| Hours after administration | Absorbed | Residue in intestine | Non-absorbed | Absorbed | Residue in intestine | Non-absorbed |
| 1 | 2.5 | 19.8 | 65.3 | -- | -- | -- |
| 2 | -- | -- | -- | 1.2 | 18.3 | 51.6 |
| 6 | 3.3 | 13.7 | 61.2 | 3.0 | 5.3 | 18.3 |
| 12 | 22.5 | 12.2 | 44.2 | 7.1 | 3.5 | 43.9 |
| 24 | 39.5 | 5.9 | 20.7 | 15.9 | 1.1 | 32.8 |
| These numbers represent the percent of the total radioactivity of each chondrotin sulfate administered orally. Note: S-labeled CS was administered orally in doseage 16 mg (2.4 x 104CPM). C-labeled CS was administered orally in doseage 3.2 mg (13.8 x 104CPM). | ||||||
In studies carried out on animals, 16 milligrams of 35S chondroitin-4-sulfate and 3.2 milligrams of 14C chondroitin- 4-sulfate were given orally to mice. About 40 percent of the 35S chondroitin-4-sulfate was absorbed within the first twenty-four hours. The ratio of absorbed material to nonabsorbed material (that remaining in the gastrointestinal tract and feces) was approximately 1:2 within twenty-four hours. The urine contained 48 percent of the 35S activity of the absorbed chondroitin sulfate. 14C chondroitin-4-sulfate was also absorbed from the intestine within twenty-four hours. Again, in the case of the 14C radioisotope, the ratio of the absorbed to nonabsorbed materials was approximately 1:2. Furthermore, high molecular weight isotope-labeled chondroitin-4-sulfate was detected in blood and in urine by electrophoretic procedures.15
TABLE XVII: ABSORPTION AND URINARY EXCRETION OF ACID MUCOPOLYSACCHARIDES (AMPS) FOLLOWING ORAL ADMINISTRATION OF CHONDROITIN SULFATE A (CSA)
| Patient | Sex | Age | Diagnosis | AMP Baseline? (mg/24hr) | AMPS After CSA (mg/24 hr) |
| T.S. | F | 43 | Coronary Heart Disease; Chronic Bronchial Asthma | 2.49 | 5.97 |
| R.L. | F | 58 | Coronary Heart Disease; Hypothyroidism | 4.20 | 6.03 |
| M.K. | F | 75 | Coronary Heart Disease; Rheumatoid Arthritis | 7.12 | 10.95 |
| L.C. | M | 69 | Generalized Arteriosclerosis; Post Myocardial Infarction | 6.79* | 5.94* |
| M.S. | F | 27 | Chronic Hepatitis; Hypothyroidism | 5.62 | 8.78 |
| L.R. | M | 54 | Coronary Heart Disease; Coronary Insufficiency | 4.78 | 6.72 |
| R.P. | F | 70 | Coronary Heart Disease | 4.48 | 6.37 |
| S.F. | M | 83 | Coronary Heart Disease; Chronic Glomerulonephritis | 5.04 | 8.09 |
| M.B. | F | 73 | Generalized Arteriosclerosis; Cardiovascular Disease; Recovered CVA | 5.16 | 6.80 |
| Statistical Average (P.01)** | |||||
| ?Average of three days determinations. Dosage levels of CSA = 10 grams daily divided into three doses with meals. | |||||
| *Doubt as to whether this patient actually ingested CSA. | |||||
| **Tests of significance were calculated according to : E. Lord, Biometrica, 37:64, 1950 and P.G. Moore, Biometrica, 44:482, 1957. | |||||
| From: L.M. Morrison and N.L. Enrick: Coronary Heart Disease: Reduction of Death Rate by Chondroitin Sulfate A, Angiology, May, 1973. | |||||
The 3H-labeled chondroitin polysulfate was given orally (400 mg/kg) to both Wistar male rats and to NewZealand male rabbits. At appropriate intervals, blood and urine samples were taken for the purpose of measuring absorbed radioactivity and breakdown products of chondroitin polysulfate. The concentrations and activities of the substances in plasma were determined by denaturing proteins (heating) and and subjecting the denatured proteins to proteolytic digestion. A filtrate was passed through a Dowex 50-X2 column (H+) and neutralized with alkali solution. Lipids were extracted with chloroform methanol.
In Figure 19, the 3H radioactivity and localization of 3H chondroitin polysulfate in paper electrophoretic patterns of rat serum at two hours after oral administration are shown.19 The fact that significantly high activity was found in an electrophoretic band corresponding to the electrophoretic mobility of 3H chondroitin polysulfate indicates that the substance was absorbed intact from intestine to circulation. Serum lipid clearing activity, measured by free fatty acid content and turbidity also appeared to be related to absorption. As can be seen in figure 20, this physiolosical activity of chondroitin polysulfate affords additional proof that chondroitin polysulfate is absorbed from the intestine.20
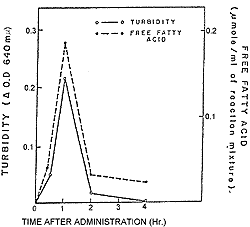 |
| Figure 20. Lipemia clearing activity of serum following oral administration of 3H chondroitin polysulfate to rabbits. |
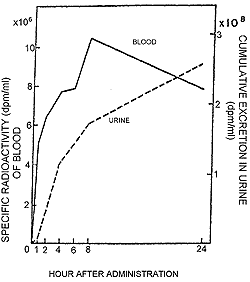 |
| Figure 21. Blood levels of radioactivity and cumulative excretion of radioactivity in urine after oral administration of 3H chondroitin polysulfate to rabbits. |
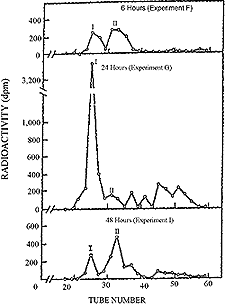 |
| Figure 22. Gel filtration proffile on Sephadex G-25 proteinase digests of rat liver following oral administration of 35S chondroitin polysulfate. |
In figure 21, the specific radioactivity in rabbit serum measured at the indicated hours after oral administration of 3H chondroitin polysulfate is shown. The blood level of radioactivity increased rapidly within the first two hours, then rose gradually to a maximum at eight hours and decreased over a period of approximately sixteen hours.21 In urine, on the other hand, a cumulative excretion of radioactivity following oral administration of 3H chondroitin polysulfate was seen.22
In a study of uptake of orally administered chondroitin sulfates in human subjects, Morrison and his co-workers23 fed individuals of a group of nine patients, displaying active symptomatic coronary heart disease in the form of angina pectoris, 10 grams of chondroitin-4-sulfate each day over a period of three days. (The dosages of chondroitin-4-sulfate were divided into three parts and were taken with meals.) Accumulated acid mucopolysaccharides excreted in the urine were measured each twenty-four hours following the initial ingestion of chondroitin-4-sulfate and were compared to a three-day baseline of urinary excretion established prior to ingestion of test polysaccharide. As can be seen in Table XVII, an increased excretion of acid mucopolysaccharides via the urine followed oral ingestion of chondroitin-4-sulfate. The average excretion of acid mucopolysaccharides during a twenty-four hour period was found to be 5.1 milligrams before patients received supplementary chondroitin-4-sulfate. During the period that 10 grams of the substance was being ingested per day, urinary excretion of total whole acid mucopolysaccharide rose to 7.3 milligrams for a twenty-four hour interval. Tests for significant differences between excretion amounts before and after administration of chondroitin-4-sulfate reveal a P value greater than 0.01.
Chondroitin polysulfate was also given orally (10 grams) to three normal subjects and the output of urinary acid mucopolysaccharides was examined over a period of seventy-two hours. The results of electrophoresis and Dowex column fractionation revealed that the increased acid mucopolysaccharide was excreted in the form of the administered chondroitin polysulfates as well as desulfated and degraded products.24
B. Distribution
In order to observe the distribution of orally administered polysulfated chondroitin sulfate among some of the internal organs of rats, Murata and his co-workers at the University of Tokyo25 used the 3H- and 35S-labeled chondroitin polysulfates employed in studies ofabsorption from the digestive tract (as described in the preceeding section). After administration, both labels were, of course, detected in the blood and organs of the digestive tract, including stomach and intestines. In the case of chondroitin polysulfate in rats, significantly high activity was detected in kidneys and liver as well as the gastro-intestinal tract. This distribution within organs is in agreement with the data of Haruki et al.26 who employed 35S chondroitin-4-sulfate in rats. The 3H-labeled chondroitin polysulfate reached a maximum in all organs between two and twelve hours after oral intake and diminished within twenty-four hours. 35S chondroitin polysulfate appeared to be absorbed more slowly than the 3H labeled material and greatest incorperation was observed between six and twenty-four hours following oral administration.It is not clear what proportion of the ingested mucopolysaccharide was present inside the cells per se, but detection of the polysaccharides in the blood and urine suggests that they must have at least reached tissues and organs, and traversed glomerular cells in order to reach this latter compartment.
C. Metabolism
As described in chapter III, enzymes are present in blood27 and tissues28 for the degradation of acid mucopolysaccharides. These include hydrolases, acetylases and desulfating enzymes.Results shown in Figure 22 are indicative of the metabolism of 35S chondroitin polysulfate in whole rat liver tissue after oral administration.29 Pronase-digested 35S mucopolysaccharides appeared in the liver within six hours but the concentration increased up to twenty-four hours when the activity of large molecular mucopolysaccharides (peak I) was predominant. At forty-eight hours, the activity of 35S was somewhat greater in peak II than in peak I.
Fractionation of urinary mucopolysaccharide preparations (obtained from rabbits receiving 35S chondroitin polysulfate) on Sephadex G-50 provided evidence of a depolymerizing action as a function of duration of metabolism.30 As can be seen in Figure 23, urine specimens taken between zero and five hours show that the larger ratio of activity appears in a high molecular fraction (A). However, the proportion of activity changes between five and eleven hours with a result that higher activity is now present in low molecular weight fractions (E, D and C). The 35S activity in each fraction diminishes gradually until only a single peak (F) is labeled from thirty-six to forty-eight hours.
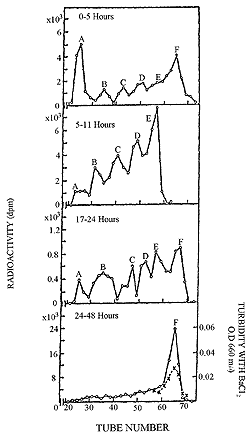 |
| Figure 23. Gel filtration on Sephadex G-50 of urine after oral administration of 35S chondroitin polysulfate. |
The above observations reveal the presence in urine of partially degraded as well as whole chondroitin polysulfates, radioactive inorganic sulfate and traces of other low molecular weight consituents (containing 35S) which are probably degadation products of chondroitin polysulfates.31
D. Excretion
As measured by Murata et al.,32 the urinary excretion of total label administered orally in the form of 35S chondroitin polysulfate and 3H chondroitin polysulfate, amounts to approximately 8 percent of the dose for the former and 7 percent of the dose for the latter over a twenty-four hour period. This attests to a significant total absorption and is in agreement with the earlier finding by Kaplan and Meyer33 that only 11 percent of chondroitin-6-sulfate injected into a dog appeared in the urine during the first day or so following injection. It is also in agreement with the report by Haruki and Murata that approximately 8 percent of nondialyzable chondroitin-4-sulfate is excreted. in man during a twenty-four hour period.34Gel filtration patterns of twelve-hour cumulative urine in rats following oral administration of 3H chondroitin polysulfate are depicted in figure 24. The highest radioactivities appeared in the void volume of Sephadex G-25 where intact high molecular chondroitin polysulfate is present. However, relatively large amounts of partially degraded substances exhibiting lower radioactivities were also detected.
E. Comment
From the above experiments, it is concluded that (a) a proportion of chondroitin sulfates given orally to animals and human subjects is absorbed in intact form (i.e. without extensive chemical changes); (b) that certain portions of the absorbed substances are distributed in various tissues and organs, but that the major portions of the absorbed substances are excreted in the urine in the form of intact or partially desulfated and depolymerized products; and (c) that acid mucopolysaccharides excreted in the urine shorty after administration show the same compositions as those of the administered chondroitin sulfates.35 It is possible that under various metabolic situations considerable differences would obtain in the amounts of chondroitin sulfates excreted in the urine. Such considerations might might vary with the age of the individual36 and the amounts and natures of tissues which are growing or being depleted of acid mucopolysaccharides at the time of administration.In tracer studies in which labeled chondroitin sulfates were employed in vivo, various amounts of degradation products were demonstrated as the result of catabolic processes. No exact explanation, however, regarding the fate of the degraded products can be presented at this time. In considering the metabolism of mucopolysaccharides, re-utilization of at least a proportion of the degraded products cannot be ruled out.
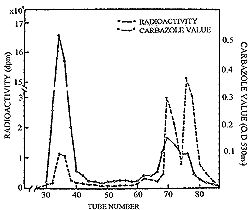 |
| Figure 24. Gel filtration on Sephadex G-25 of twelve hours cumulative urine following oral administration of 3H chondroitin polysulfate to rats. |
Thus far, it remains to be unequivocally that chondroitin sulfates actually enter all cells and if they do, whether some cells are more receptive to them than others. In this regard, it is pertinent to point out that even though biological effects are observed with both injected and orally administered chondroitin sulfates, this is no reason for assuming that they enter all of the cells showing reactions to them. In the scheme of bone induction, as experimentally elucidated by Urist and others,37 it appears that the inducing agent is probably a complex of mucopolysaccharides in associntion with a bone tropocollagen which never enters the presumptive osteoblasts. All of the evidence (including labeling with radioactive materials) indicates that no uptake of the inducing agent by the induced cell takes place.38 In this case the induction process appears to consist of contact of the inducing agent with the surface of a cell which is differentiated in the direction of responding to such a stimulus.
It is, of course, neccessary to learn the nature of the effects of mucopolysaccharidess at the intracellular level if controlled and intelligent therapy is to be applied. For this reason, much important study remains to be carried out with respect to the possible entry of chondroitin sulfates into various cell types, how they enter, if they do and the reactions which occur in the cell thereafter, especially at the level of the genome. Investigations involving the use of radioactive chondroitin-4-sulfate, etc., in tissue cultures and other systems, followed by cell fractionation and analysis of such fractions, would appear to be useful in this regard, as would studies involving radioautography at the level of the electron microscope.
Notes.
1E. De Robertis, and A. Vaz Ferreira, Submicroscopic changes of the nerve endings in the adrenal medulla after stimulation of the splanchnic nerve. J. Biophys Biochem Cytol, 3:611, 1957.2H. Blaschka, A.D. Smith, H. Winkler, H. Van den Borsch, and L.L. Van Deene, Acid phospholipase A in lysosomes of the bovine adrenal medulla. Biochem J, 103:30-32c, 1967.
3F.H. Schneider, A.D. Smith, and H. Winkler, Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol, 31:94, 1967.
4F.W.R. Brambell, W.A. Hemmings, C.C. Oakley, and R.R. Porter, The relative transmission of the fractions of papain hydrolyzed homologous globulin from the uterine cavity to the portal circulation of the rabbit. Proc R Soc Lond (Biol), 151:478, 1960.
5O.A. Schjeide, F. Galey, E.A. Grellert, R. I-San-Lin, J. de Vellis, and J.F. Meade, Macromolecles in oocytes maturation. Biol Reprod (supp),2:14, 1970.
6C.P. Triganoo, and H. Muir, Studies on protein polysaccharides from pig laryngeal cartilage. Hetrogeneity, fractionation and characterization. Biochem J, 113:855, 1969.
7C.M. Feldherr, Nucleocytoplasmic exchanges during cell division. J Cell Biol, 31:199, 1966.
8C.M. Feldherr, The effect of electron-opaque pore material on exchanges through the nuclear annuli. J Cell Biol, 31:199, 1966.
9Feldherr, The effect of the electron opaque pore material on exchanges through the nuclear annuli.
10Feldherr, Nucleocytoplasmic exchanges during cell division.
11L.B. Crittenden, W.E. Briles, and H.A. Stone, Susceptability to an avian lukosis-sarcoma virus: a close association with erythrocyte isoantigen. Science, 59:1324, 1970.
12K. Murata, M. Nomato, and T. Funaki, In preparation.
13F. Haruki, K. Murata, and H. Debuchi, Further studies on the metabolism of chondroitin sulfate. Jap Rheum, 2:453, 1960.
14D.D. Dziewiatowski, Some aspects of the metabolism of chondroitin sulfate-35S in the rat. J Biol Chem,223:239, 1956.
15K. Murata, Chondroitin sulfates in clinical aspects. In Biochemistry of Acid Mucopolysaccharides, S. Suzuki, T. Matsumura, and I. Yamashina, Eds. (Tokyo, Kyoritsu, 1970), vol. II, p. 1140.
16T. Ofuji, and T. Funaki, Absorption and excretion of sodium chondroitin polysulfate. In preparation.
17Ibid.
18Murata, Nomoto, and Funaki.
19Ibid.
20Ibid.
21Ibid.
22Ibid.
23L.M. Morrison, P.G. Rucker, and M.R. Stevens, Unpublished observations.
24T. Ogura, S. Suzuki, and T. Yamaguchi, Studies on urinary mucosubstances following chondroitin polysulfate administration in men. In preparation.
25Murata, Nomoto, and Funaki.
26Haruki, Murata, and Debuchi.
27M.L. Salkie, The acid glycosaminoglycan-degrading activity of normal human serum. Clin Chim Acta, 31:300, 1971.
28N.N. Aronson, Jr., and E.A. Davidson, Catabolism of mucopolysaccharides by rat liver lysosome in vivo. J Biol Chem, 243:4494, 1968.
29Murata, Nomoto, and Funaki
30Ibid.
31Ibid.
32Ibid.
33D. Kaplan, and K. Meyer, The fate of injected mucopolysaccharides. J Clin Invest, 41:743, 1962.
34Haruki, Murata, and Debuchi.
35Murata, Nomoto, and Funaki.
36Ibid.
37M.R. Urist, Induction and differentiation of cartilage and bone cells. In: Cell Differentiation, O.A. Schjeide, and J. De Vellis, Eds. (New York, Van Nostrand Reinhold, 1970), p.504.
38Ibid.