Oral Administration of Ascorbic Acid to Horses | Equine Clinical Research
EQUINE VETERINARY JOURNAL (1987) 19(6), 520-523
Reprinted with permission
A group of six healthy Thoroughbred geldings aged between four and 12 years (weight 434 kg to 512 kg) were used for oral and iv administrations of ascorbic acid. For the iv administration 10 g of ascorbic acid (BDH Biochemicals, Poole, Dorset) was dissolved in a solution containing 4.8 g sodium bicarbonate, made up to 100 ml and filtered through a 0.2 F m filter prior to injection into the jugular vein of three horses through a catheter over a period of 5 mins. Blood samples were collected from the other jugular prior to administration and 0.08, 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10h thereafter.
For oral administration either a single dose was given for which 10 g of ascorbic acid was dissolved in a solution containing 4.8 g sodium bicarbonate to give a final volume of 1 litre. This was then administered after an overnight fast to the three horses used in the iv study. Blood samples were taken at similar intervals as for the iv administration. The horses were given their normal feed 3 h after dosing.The investigation on repeated daily administration was carried out on six horses using a cross-over design, with three horses being supplemented and three unsupplemented with ascorbic acid. Before ascorbic acid administration in the first three horses, five blood samples were collected over an eight day period to establish baseline concentration. Then 20 g of crystalline ascorbic acid, analysed as being 99 per cent active ascorbic acid, was given daily for a period of 25 days. The ascorbic acid was administered by mixing it in a small amount of bran fed in the evening, followed by their normal feed of 3.8 kg commercial horse cubes (racehorse cubes: Spillers) and 2.5 kg hay. Another three unsupplemented horses acted as controls over this period.Following the first 25 days, three control horses were then supplemented with 20 g daily for the same period, while the previously supplemented acted as controls. All six horses had regular blood samples taken at 09.00 h during the 65 day period (Fig 3).
Between stable studies (Thoroughbred flat racing)
In Stable A, all 140 horses were being supplemented with ascorbic acid, 20 g being given daily by mixing, together with other supplements in the morning feed. From this group 19 horses were sampled. The daily diet in this stable was oats and approximately 7 kg English and American hay.No ascorbic acid supplementation was used in Stable B. Of the 50 horses, 25 were sampled. The daily diet in this stable was a combination of oats (3.5 kg) and racehorse cubes (4.2 kg) (Spillers) plus English and American hay.In both Stables A and B, blood samples were taken at approximately monthly intervals during the period February to October. Because of racing commitments, culling, etc, not all horses could be sampled on every occasion.Fourteen horses in Stable C were sampled before and following 27 days of oral administration with 4.5 kg ascorbic acid given five days per week.In Stables A, B and C, blood was collected between 16.30 h and l8.00 h and before evening feedings.
Sample preparation
Venous blood was collected by syringe and transferred into 10 ml plastic tubes containing 0.2 ml solution of 0.25 M ethylene glycol tetraacetic acid (EGTA) and 0. 195 M reduced glutathione (GSH) as anticoagulant and reducing agent, respectively. The plasma was removed within 1 h and stored at -45 E C(stability at least 10 months) prior to analysis. Any thawing and refreezing was found to result in appreciable loss of activity.
On thawing, an 0.75 ml aliquot was immediately extracted by mixing with 20 per cent metaphosphoric acid (0.25 ml) containing 5 mM EGTA and 3.8 mM GSH. After centri-fugation at 9000 g for 5 mins the supernatant was left refrigerated for between 12 h and 24 h to ensure complete reduction of dehydroascorbic acid to L ascorbic acid. These extracts were found to be stable for 48 h at room temperature, at least 14 days at 4 E C and at least six months at -45 EC.
Immediately before chromatography, 50F l aliquots of the extract were buffered to pH 3 with 25 F l 1M sodium potassium phosphate pH 8.5; 20 F l was used for chromatography.
Spiked plasma samples were used as standards. To remove endogenous ascorbic acid, 23 ml plasmawastirred for two days at room temperature with 1 ml 20 volumes hydrogen peroxide. Catalase (23 F l) (approximately 40,000 iu) was then added and the mixture stirred at room temperature for 6 h before being refrigerated overnight. Using this plasma, standards of 0, 1, 5, 10 and 20 F g/ml were made. From each concentration aliquots of 0.75 ml were taken, extracted with 0.2 ml 20 per cent metaphosphoric acid containing EGTA and GSH.
The supernatants were left refrigerated overnight and 50 Fl aliquots frozen and stored at -45 C. For each HPLC run, these aliquots were used to establish the standard curve.
Chromatographic analysis was carried out by HPLC using a modification of the methods by Bui-Nguyen (1980) and Rose and Nahrwold (1981). Ion-modified partition chromatography was carried out on a column 250 mm x 4.6 mm packed with Spherisorb NH2 5Fl preceded by a guard column 60 mm x 3 mm of pellicular polar bonded phase 30 to 40 Fl material. The mobile phase was 75 per cent acetonitrile 25 per cent 50 mM potassium phosphate buffer at pH 5.2 delivered at ambient temperature isocratically at 1.0 ml/min using an LDC IIG Constametric pump. Samples were injected via a Rheodyne 7125 valve with a 20Fl loop. Peak heights were determined at 268 nm using a Pye Unicam LC UV detector and a Tekman chart recorder set at 1 cm/min.
It was necessary to repack the guard column after approximately 300 injections. Occasionally, a reduction in plate count in the column was noticed. This occurred quite suddenly and remained unaffected by repacking the guard column. Total recovery of plates was achieved by washing the analytical column at 0.5 ml/min with 50 mM ethanolamine buffered to pH 7.4 with 50 mM citric acid. The limit of detection for the method was 0.1 Fg/ml ascorbic original plasma concentration.To confirm specificity of the ascorbic acid peak, some spiked plasma samples were treated prior to extraction with ascorbate oxidase. To confirm the precision of the assay, samples were assayed using this method and in another laboratory where a fluorimetric method (Brubacher and Vuillemier 1974) is used. For 43 samples a multiple correlation coefficient of 0.91 and a line of best fit of y = 0.2675 + 0.8802x for the fluorimetric method versus HPLC were achieved.Data analysisThe calculation of pharmacokinetic data from iv administered ascorbic acid was performed using a PC Lin programme. Significance between treatments and monthly variations was tested using a Student's t test. Results are given as mean sd.
Intravenous Administration
For comparative purposes the mean result from the three horses was analysed using the two-compartment open model as described in detail by Loscher et al (1984). The mean plasma curve is shown in Fig 2 and calculated pharmacokinetic parameters shown in Table I. Results were calculated without subtracting the baseline value of ascorbic acid.Oral administration Single dose. - As can be seen from Fig 3, there was no appreciable increase in plasma ascorbic acid in the first 10 h after administration.
Repeated daily administration. - In all six horses there was a significant increase (P<0.01) in plasma ascorbic acid concentrations, when mean values during treatment are compared to those prior to or following cessation of supplementation. The results for two horses are shown in Fig 4. Mean value without supplementation was 2.7 + 1.4~R/ml and with supplementation 5.9 + 1.5 F/ml.
Between stable studies.- Table 2 shows that plasma ascorbic acid concentrations were significantly higher (P<0.001) in the supplemented stable (Stable A) than in the unsupplemented stable (Stable B). In addition it appears that monthly variations in plasma concentrations can occur. In Stable C, mean plasma ascorbic acid concentration was significantly increased (P<0.01) from 4.7" 1.3 F g/ml to 7.7 >" 1.9 F g/ml after supplementation.
Discussion Before determining plasma ascorbic acid concentration by HLPC, a rapid colorimetric method was tried (Jagota and Dani 1982) but was found to be unsatisfactory. The method ussed in this investigation was specific and gave similar results to a fluorimetric method.Acomparison of the intravenous results with those reported by Loscher et al (1984) for 10 g given to heavy horses shows some differences. Both the half-lives of the fast and slow disposition curves were more rapid in this present study. Whether this represents breed or methodological differences cannot be ascertained, because the breeds used in the Loscher study are not specified. The 5 mins taken for injection of the ascorbic acid could have contributed to the half-life of the fast curve. The size of the central compartment is similar to that previously reported and approximately equivalent to the extracellular body water. However, the apparent volume of distribution at pseudodistribution and that at a steady state of equilibrium between the peripheral and central compartments are higher. This suggests a greater extent of tissue binding than reported by Loscher et al (1984).
The major result from this study is the finding, in agreement with previous studies (Errington, Hodgkiss and Jayne 1942; Loscher et al 1984), that very poor absorption of ascorbic acid occurs after oral administration. However, repeated administration does increase plasma concentrations. Interestingly, the increase in plasma concentrations following repeated administration is similar for both 4.5 g and 20 g doses. Why an approximate doubling of plasma concentrations after continuous dosing and the little difference between the two dose rates occurs is presently unknown, and further studies are necessary to investigate these aspects. The plasma ascorbic acid concentrations in unsupplemented horses in this study are similar to those reported by Pearson, Sheybani and Schmidt (1943), Stillions, Teeter and Nelson (1971), and lower than that described by Errington et al (1942) and Loscher et al (1984). It is the latter two groups that reported no appreciable increase in plasma ascorbic acid concentrations following oral administration. It is, therefore, possible that their higher concentrations may have been because of a lack of specificity of their colorimetric method, thus masking small increases following oral administration. The mean plasma concentrations in unsupplemented horses found in this present study are within the range of 2.0 to 4.0 F g/ml, which is reported in man to be marginally adequate (Kallner, Hartmann and Hornig 1979). Unfortunately whether this is the case in horses is unknown. However, as shown in the study, supplementation will raise plasma concentrations out of this range. Ascorbic acid has a number of functions in the body, the major one being the formation of connective tissue. It has also been suggested to be important in helping to withstand both physiological and pathological stresses (Thaxton and Pardue 1984). For example, supplementation with ascorbic acid has been beneficial to chickens exposed to high environmental temperatures (Pardue, Thaxton and Brake 1985).From studies in both the supplemented and unsupplemented stables, it is interesting to note that there is a trend for lowest plasma ascorbic acid concentrations to occur in March and April, a time when these Thoroughbred horses would have been exposed to the greatest physiological stress as they were entering into hard work. It is considered unlikely that dietary differences would contribute to these fluctuations.Further studies on possible monthly fluctuations because of training or disease are being undertaken to see if there may be a place for ascorbic acid supplementation in horses. However, these present studies do indicate that, contrary to the assertions of Loscher et al (1984), oral administration is a suitable route for supplementation to raise systemic ascorbic acid concentrations. The most appropriate dose rate still has to be ascertained; however, from a safety aspect it would appear that 20 g daily can be given without any untoward effect. The stable using the 20 g dose rate is one of the most successful in the country, and no apparent deleterious effects can be attributed to this or any other supplementation over several years of administration.
The experimental horses were kindly looked after by P. Ferrie and T. Redworth. Fluorimetric analysis of some plasma samples was carried out by F. Hoffman-La Roche, Basle, and assistance in pharmacokinetic analyses provided by Dr. G. Lockwood, NAPP Research Centre, Cambridge.
Brubacher, G. and Vuillemier, J. P. (1974) Vitamin C. In: Clinical Biochemistry Principles and Methods, Vol 2. Eds. H. Ch. Curtius and M. Roth. Walter de Gruyter, Berlin, New York, pp 989-997. Bui-Nguyen, M. H. (1980) Application of high performance liquid chromatography to the separation of ascorbic acid from isoascorbic acid. J. Chromat. 196, 163-165.ErrinLton, B. J., Hodgkiss, W. S. and Jayne, E. P. (1942) Ascorbic acid in certain body fluids of horses. Am. J. Vet. Res. 3, 242. Jaeschke, G. ( 1984) Influence of ascorbic acid on physical development and performance of racehorses. Proc. Workshop on Ascorbic Acid in Domestic Animals, Scan. Ass. Agr. Sci. and Royal Dan. Agr. Soc. Eds. I. Wegger, F. J. Tagwerker and J. Moustgaard. pp 153-161.Jaeschke, G. and Keller. H. (1978) Beitraz zum Ascorbinsaurestatus des Pferdes. 2. Mitteiling Klinische Aspekte und Mangelsituationen. Berl. Munch. Tierazrl. Wschr. 91, 375-379.Jagota, S. K. and Dani, H. M. (1982) A new colorimetric technique for the estimation of Vitamin C using Folin Phenol Reagent. Analyt. Biochem. 127, 178-182.Kallner, A., Hartmann, D. and Hornig, D. (1979) Steady-state turnover and body pool of ascorbic acid in man. Am. J. Clin. Nutr. 32, 530-539.Loscher, W., Jaeschke, G. and Relier, H. (1984) Pharmacokinetics of ascorbic acid in horses. Equine Vet. J. 16, 59-65.Pardue, S. L., Thaxton, J. P. and Brake, J. (1985) Role of ascorbic acid in chicks exposed to high environmental temperature. J. Appl. Physiol. 58, 1551-1516.Pearson, P. B., Sheybani, M. K. and Schmidt, H. (1943) The metabolism of ascorbic acid in the Horse. J. Anim. Sci. 2, 175-180. Rose, R. C, and Nahrwold, D. L. (1981) Quantitative analysis of ascorbic acid and dehydroascorbic acid by high performance liquid chromatography. Analyt. Biochem. 114, 140-145. Stillions, M. C., Teeter, S. M. and Nelson, W. E. (1971) Ascorbic acid requirements of mature horses. J. Anim. Sci. 32, 249-251. Thaxton, J. P. and Pardue, S. L. (1984) Ascorbic acid and physiological stress. Proc. Workshop on Ascorbic Acid in Domestic Animals, Scand Ass. Agr. Sci and Royal Dan. Agr. Soc. Eds 1. Wegger, F., T.,Tagwerker and J. Moustgaard. p 153-161.
Reprinted with permission
Oral Administration of Ascorbic Acid to Horses
D. H. SNOW, S. P. GASH and J. CORNELIUS Physiology Unit, The Animal Health Trust, Snailwell Road, Newmarket, Suffolk CB8 7DW
Summary
The effects of oral administration of high doses of ascorbic acid on plasma concentrations were investigated in both experimental Thoroughbred horses and those within racing stables. A single oral dose (20 g) did not result in any increase in plasma concentrations. However, daily administration of either 4.5 g or 20 g doses resulted in significant increases in plasma concentrations. Monthly variations in plasma ascorbate concentrations were found in both supplemented (20 g daily) and unsupplemented stables. It is concluded that oral supplementation with ascorbic acid is a satisfactory route to increase plasma and tissue concentrations.Introduction
In a number of infections plasma ascorbic acid concentration is reduced below normal (Jaeschke and Keller 1978, Jaeschke 1984). From these findings they have suggested that there may be a place for ascorbic acid supplementation in the horse, despite it being able to synthesise the compound. In a study of the best means of supplementing with ascorbic acid, it was reported that, following oral administration, systemic availability was very poor (Loscher, Jaeschke and Keller 1984). Because subcutaneous and intramuscular (im) administration resulted in marked local irritation, it was concluded that intravenous (iv) injection was the only satisfactory route of administration.A number of leading trainers in England are administering high doses of ascorbic acid orally to their horses. It was, therefore, decided to carry out further studies on its biological availability. This paper reports on preliminary findings, involving a specific high performance liquid chromatography (HPLC) method, which indicates that plasma concentrations of ascorbic acid increase following repeated oral administration.Materials and methods
Experimental studiesA group of six healthy Thoroughbred geldings aged between four and 12 years (weight 434 kg to 512 kg) were used for oral and iv administrations of ascorbic acid. For the iv administration 10 g of ascorbic acid (BDH Biochemicals, Poole, Dorset) was dissolved in a solution containing 4.8 g sodium bicarbonate, made up to 100 ml and filtered through a 0.2 F m filter prior to injection into the jugular vein of three horses through a catheter over a period of 5 mins. Blood samples were collected from the other jugular prior to administration and 0.08, 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10h thereafter.
For oral administration either a single dose was given for which 10 g of ascorbic acid was dissolved in a solution containing 4.8 g sodium bicarbonate to give a final volume of 1 litre. This was then administered after an overnight fast to the three horses used in the iv study. Blood samples were taken at similar intervals as for the iv administration. The horses were given their normal feed 3 h after dosing.The investigation on repeated daily administration was carried out on six horses using a cross-over design, with three horses being supplemented and three unsupplemented with ascorbic acid. Before ascorbic acid administration in the first three horses, five blood samples were collected over an eight day period to establish baseline concentration. Then 20 g of crystalline ascorbic acid, analysed as being 99 per cent active ascorbic acid, was given daily for a period of 25 days. The ascorbic acid was administered by mixing it in a small amount of bran fed in the evening, followed by their normal feed of 3.8 kg commercial horse cubes (racehorse cubes: Spillers) and 2.5 kg hay. Another three unsupplemented horses acted as controls over this period.Following the first 25 days, three control horses were then supplemented with 20 g daily for the same period, while the previously supplemented acted as controls. All six horses had regular blood samples taken at 09.00 h during the 65 day period (Fig 3).
Between stable studies (Thoroughbred flat racing)
In Stable A, all 140 horses were being supplemented with ascorbic acid, 20 g being given daily by mixing, together with other supplements in the morning feed. From this group 19 horses were sampled. The daily diet in this stable was oats and approximately 7 kg English and American hay.No ascorbic acid supplementation was used in Stable B. Of the 50 horses, 25 were sampled. The daily diet in this stable was a combination of oats (3.5 kg) and racehorse cubes (4.2 kg) (Spillers) plus English and American hay.In both Stables A and B, blood samples were taken at approximately monthly intervals during the period February to October. Because of racing commitments, culling, etc, not all horses could be sampled on every occasion.Fourteen horses in Stable C were sampled before and following 27 days of oral administration with 4.5 kg ascorbic acid given five days per week.In Stables A, B and C, blood was collected between 16.30 h and l8.00 h and before evening feedings.
Sample preparation
Venous blood was collected by syringe and transferred into 10 ml plastic tubes containing 0.2 ml solution of 0.25 M ethylene glycol tetraacetic acid (EGTA) and 0. 195 M reduced glutathione (GSH) as anticoagulant and reducing agent, respectively. The plasma was removed within 1 h and stored at -45 E C(stability at least 10 months) prior to analysis. Any thawing and refreezing was found to result in appreciable loss of activity.
On thawing, an 0.75 ml aliquot was immediately extracted by mixing with 20 per cent metaphosphoric acid (0.25 ml) containing 5 mM EGTA and 3.8 mM GSH. After centri-fugation at 9000 g for 5 mins the supernatant was left refrigerated for between 12 h and 24 h to ensure complete reduction of dehydroascorbic acid to L ascorbic acid. These extracts were found to be stable for 48 h at room temperature, at least 14 days at 4 E C and at least six months at -45 EC.
Immediately before chromatography, 50F l aliquots of the extract were buffered to pH 3 with 25 F l 1M sodium potassium phosphate pH 8.5; 20 F l was used for chromatography.
Spiked plasma samples were used as standards. To remove endogenous ascorbic acid, 23 ml plasmawastirred for two days at room temperature with 1 ml 20 volumes hydrogen peroxide. Catalase (23 F l) (approximately 40,000 iu) was then added and the mixture stirred at room temperature for 6 h before being refrigerated overnight. Using this plasma, standards of 0, 1, 5, 10 and 20 F g/ml were made. From each concentration aliquots of 0.75 ml were taken, extracted with 0.2 ml 20 per cent metaphosphoric acid containing EGTA and GSH.
The supernatants were left refrigerated overnight and 50 Fl aliquots frozen and stored at -45 C. For each HPLC run, these aliquots were used to establish the standard curve.
Chromatographic analysis was carried out by HPLC using a modification of the methods by Bui-Nguyen (1980) and Rose and Nahrwold (1981). Ion-modified partition chromatography was carried out on a column 250 mm x 4.6 mm packed with Spherisorb NH2 5Fl preceded by a guard column 60 mm x 3 mm of pellicular polar bonded phase 30 to 40 Fl material. The mobile phase was 75 per cent acetonitrile 25 per cent 50 mM potassium phosphate buffer at pH 5.2 delivered at ambient temperature isocratically at 1.0 ml/min using an LDC IIG Constametric pump. Samples were injected via a Rheodyne 7125 valve with a 20Fl loop. Peak heights were determined at 268 nm using a Pye Unicam LC UV detector and a Tekman chart recorder set at 1 cm/min.
It was necessary to repack the guard column after approximately 300 injections. Occasionally, a reduction in plate count in the column was noticed. This occurred quite suddenly and remained unaffected by repacking the guard column. Total recovery of plates was achieved by washing the analytical column at 0.5 ml/min with 50 mM ethanolamine buffered to pH 7.4 with 50 mM citric acid. The limit of detection for the method was 0.1 Fg/ml ascorbic original plasma concentration.To confirm specificity of the ascorbic acid peak, some spiked plasma samples were treated prior to extraction with ascorbate oxidase. To confirm the precision of the assay, samples were assayed using this method and in another laboratory where a fluorimetric method (Brubacher and Vuillemier 1974) is used. For 43 samples a multiple correlation coefficient of 0.91 and a line of best fit of y = 0.2675 + 0.8802x for the fluorimetric method versus HPLC were achieved.Data analysisThe calculation of pharmacokinetic data from iv administered ascorbic acid was performed using a PC Lin programme. Significance between treatments and monthly variations was tested using a Student's t test. Results are given as mean sd.
Results
A typical chromatograph before and after ascorbate oxidase treatment is shown in Fig 1. The complete reduction of dehydroascorbic acid to ascorbic acid meant that total active forms of vitamin C were determined. Retention time for ascorbic acid was 12 mins.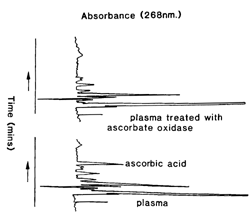 |
| Fig 1. HPLC profiles of metaphosphoric acid extracts collected into EGTA/GSH, with and without incubation at 37° C for 2 h with ascorbate oxidase. |
For comparative purposes the mean result from the three horses was analysed using the two-compartment open model as described in detail by Loscher et al (1984). The mean plasma curve is shown in Fig 2 and calculated pharmacokinetic parameters shown in Table I. Results were calculated without subtracting the baseline value of ascorbic acid.Oral administration Single dose. - As can be seen from Fig 3, there was no appreciable increase in plasma ascorbic acid in the first 10 h after administration.
Repeated daily administration. - In all six horses there was a significant increase (P<0.01) in plasma ascorbic acid concentrations, when mean values during treatment are compared to those prior to or following cessation of supplementation. The results for two horses are shown in Fig 4. Mean value without supplementation was 2.7 + 1.4~R/ml and with supplementation 5.9 + 1.5 F/ml.
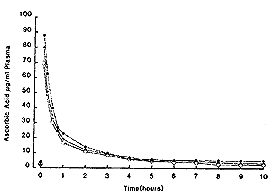 |
| Fig. 2. Plasma ascorbic acid concentrations in 3 horses following 10 g ascorbic acid iv. |
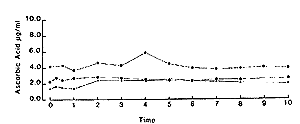 |
| Fig. 3. Plasma ascorbic acid concentrations in 3 horses following oral administration of 10 g ascorbic acid to fasted animals. |
| TABLE 1: Pharmacokinetic parameters of ascorbic acid in horses derived from two-compartment moel analysis of plasma data after administration of 10 g ascorbic acid. Results were obtained from the data of three horses (mean" sd) | |||
| Kinetic term | Units | Present findings | Heavy horse* |
| Bodyweight | kg | 479"55 | |
| Cp | F g/ml | 95.4 " 10.0 | 110 |
| A | F g/ml | 78.4 " 10.6 | 91 |
| a | h-1 | 2.8 " 0.3 > | 1.49 |
| t 2 ( a ) | h | 0.25 " 0.02 | 0.56 |
| B | F g/ml | 17.0 " 0.20 | 20 |
| b | h-1 | 0.20 " 0.04 | 0.085 |
| t 2 ( b ) | h | 3.60 " 0.63 | 8.7 |
| k12 | h-1 | 1.49 " 0.11 | 0.78 |
| k21 | h-1 | 0.67 " 0.15 | 0.33 |
| k10 | h-1 | 0.83 " 0.06 | 0.38 |
| V1 | litre/kg | 0.22 " 0.01 | 0.19 |
| V2 | litre/kg | 0.50 " 0.08 | 0.46 |
| Vd( b ) | litre/kg | 1.01 " 0.17 | 0.79 |
| Vd(ss) | litre/kg | 0.72 " 0.08 | 0.65 |
| Cltot) | ml/kg/min | 3.02 " 0.18 | 1.04 |
| CpZero time serum level; a zero time drug conc. Intercept of fast dsposition curve; B Zero time drug conc. Intercept of fast disposition curve; a Fast disposition rate constant; b slow disposition rate constant; t 2FONT face=Symbol>a ) Plasma half life of fast disposition curve; t 2 ( b >) Plasma half life of slow disposition curve; k12First order rate constant for drug distribution between the central and peripheral compartments; V1 Apparent volume of central compartment; V2 Apparent volume of peripheral compartment; Vd(b) Apparent volume of distribution at pseudodistributionequilibrium; Dd(ss) Apparent volume of distribution at a steady state of equilibrium between V1 and V2; Cl(tot) Total body clearance. | |||
| * From Loscher et al (1984) | |||
Between stable studies.- Table 2 shows that plasma ascorbic acid concentrations were significantly higher (P<0.001) in the supplemented stable (Stable A) than in the unsupplemented stable (Stable B). In addition it appears that monthly variations in plasma concentrations can occur. In Stable C, mean plasma ascorbic acid concentration was significantly increased (P<0.01) from 4.7" 1.3 F g/ml to 7.7 >" 1.9 F g/ml after supplementation.
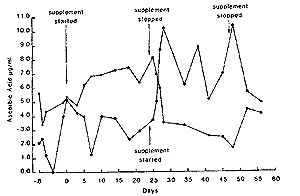 |
| Fig. 4. Plasma ascorbic acid concentrations in 2 horses with and without supplementation of ascorbic acid daily. |
TABLE 2: Comparison of plasma ascorbic acid concentrations in two racing stables
| |||||||
| Month | n | Mean | sd | n Mean | sd | ||
| February | 14 | 5.6 | 2.3 | 21 2.7 | 1.0 | ||
| March | 18 | 4.1 | 1.6 | 23 2.6 | 1.0 | ||
| April | 18 | 4.7 | 1.5 | 35 2.0a** | 0.9 | ||
| May | 17 | 6.8a**** | 1.4 | 24 3.0*** | 1.2 | ||
| June | 16 | 6.7 | 3.1 | 24 2.6 | 1.0 | ||
| July | 17 | 7.6 | 2.6 | 24 3.2 | 1.8 | ||
| August | 16 | 8.3 | 3.6 | 19 4.2 | 1.5 | ||
| September | 14 | 5.5*** | 0.9 | 20 2.8*** | 1.1 | ||
| October | 13 | 5.0 | 1.5 | 8 2.5 | 0.6 | ||
| A Comparison to previous month's plasma ascorbic acid concentration. | |||||||
| * = P>0.05, ** = P>0.02, *** P <0.01, **** = P <0.0001 | |||||||
Acknowledgements
The experimental horses were kindly looked after by P. Ferrie and T. Redworth. Fluorimetric analysis of some plasma samples was carried out by F. Hoffman-La Roche, Basle, and assistance in pharmacokinetic analyses provided by Dr. G. Lockwood, NAPP Research Centre, Cambridge.
References
Brubacher, G. and Vuillemier, J. P. (1974) Vitamin C. In: Clinical Biochemistry Principles and Methods, Vol 2. Eds. H. Ch. Curtius and M. Roth. Walter de Gruyter, Berlin, New York, pp 989-997. Bui-Nguyen, M. H. (1980) Application of high performance liquid chromatography to the separation of ascorbic acid from isoascorbic acid. J. Chromat. 196, 163-165.ErrinLton, B. J., Hodgkiss, W. S. and Jayne, E. P. (1942) Ascorbic acid in certain body fluids of horses. Am. J. Vet. Res. 3, 242. Jaeschke, G. ( 1984) Influence of ascorbic acid on physical development and performance of racehorses. Proc. Workshop on Ascorbic Acid in Domestic Animals, Scan. Ass. Agr. Sci. and Royal Dan. Agr. Soc. Eds. I. Wegger, F. J. Tagwerker and J. Moustgaard. pp 153-161.Jaeschke, G. and Keller. H. (1978) Beitraz zum Ascorbinsaurestatus des Pferdes. 2. Mitteiling Klinische Aspekte und Mangelsituationen. Berl. Munch. Tierazrl. Wschr. 91, 375-379.Jagota, S. K. and Dani, H. M. (1982) A new colorimetric technique for the estimation of Vitamin C using Folin Phenol Reagent. Analyt. Biochem. 127, 178-182.Kallner, A., Hartmann, D. and Hornig, D. (1979) Steady-state turnover and body pool of ascorbic acid in man. Am. J. Clin. Nutr. 32, 530-539.Loscher, W., Jaeschke, G. and Relier, H. (1984) Pharmacokinetics of ascorbic acid in horses. Equine Vet. J. 16, 59-65.Pardue, S. L., Thaxton, J. P. and Brake, J. (1985) Role of ascorbic acid in chicks exposed to high environmental temperature. J. Appl. Physiol. 58, 1551-1516.Pearson, P. B., Sheybani, M. K. and Schmidt, H. (1943) The metabolism of ascorbic acid in the Horse. J. Anim. Sci. 2, 175-180. Rose, R. C, and Nahrwold, D. L. (1981) Quantitative analysis of ascorbic acid and dehydroascorbic acid by high performance liquid chromatography. Analyt. Biochem. 114, 140-145. Stillions, M. C., Teeter, S. M. and Nelson, W. E. (1971) Ascorbic acid requirements of mature horses. J. Anim. Sci. 32, 249-251. Thaxton, J. P. and Pardue, S. L. (1984) Ascorbic acid and physiological stress. Proc. Workshop on Ascorbic Acid in Domestic Animals, Scand Ass. Agr. Sci and Royal Dan. Agr. Soc. Eds 1. Wegger, F., T.,Tagwerker and J. Moustgaard. p 153-161.